Using AI for Life Sciences R&D
The current state of drug development
Bringing any treatment to market is a multi-stage, multi-team effort. Starting with the scientists during drug discovery and ending with the stats and regulatory teams prepping a regulatory submission. This journey requires effective and efficient execution – there are many processes and spots where things can go awry. This acknowledgment of failure is built into the drug development framework – we know that we must have a lot of drugs in the pipeline to get to a single approval, as seen in the image below.
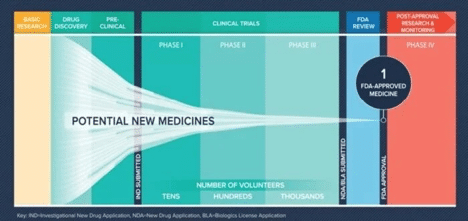
If we dive a little deeper into the development funnel we come across some sobering stats…

If we look at the odds of a particular drug candidate being approved, we see a relatively small success rate of 9.6%. This isn’t a great number, but it looks like amazing success when you consider the odds of developing a blockbuster drug (a drug that brings in $1B or more for an organization) at a mere 0.1%.
These odds of success (or failure, depending on how you look at it) are exacerbated by the fact that it takes, on average, 14 years to bring a treatment to market. This means we won’t know for over a decade if we made the right decisions during the discovery phase.
If you multiply the success rate and the duration of bringing a drug to market, we land on the level of investment a Life Sciences firm has to make to bring the average drug to market – $2.3 billion.
Drug development optimization goals
While I wrote earlier that the industry had acknowledged this funnel (and investment) paradigm, they are not content with the current state. Every R&D leader I talk to is focused on doing better in the name of bringing needed treatments to underserved patients.

These industry leaders are focused on two main parts of the drug development funnel.
- The first is looking to increase the odds of success that identified and researched candidates reach and receive regulatory approval. This allows a company to invest more in the treatments they have confidence will be successful.
- The second is looking to decrease the time-to-market (duration from candidate identification to regulatory approval.) This reduces development costs and preserves patent exclusivity periods. Both of these things allow a company to invest in more discovery to drive even more innovation hopefully.
Traditional approaches to optimization
So now that we know that Life Science companies have goals of improvement, how have they attempted to achieve these improvements? Pharma companies have tried a few approaches to optimize the process of bringing treatments to market.
- First, they have created partnerships with CROs and other organizations. These partnerships give the Life Sciences company access to expertise and experience that is hopefully valuable in improving process execution.
- Next, they have a heavy focus on evaluating processes (in particular for clinical trial execution where a Phase III trial alone can cost tens to hundreds of millions of dollars) and looking to see how they can streamline the processes – more and more that involves taking a risk-based approach (like risk-based monitoring) when possible.
- Last but certainly not least, they have been investing in systems that support particular business processes. These applications may range from site selection and activation tools to data management tools to regulatory submission publishing tools.
These initiates and investments have improved individual process execution, but the cost and duration of drug development aren’t dropping as these leaders would hope. We are getting to a point of diminishing returns for these foundational initiatives, provided that a company has pursued them all…something else needs to step in and help.
The modern approach to optimization
Pharma leaders are now viewing data as a key differentiator and the ability to leverage that data as a way to make significant improvements in bringing drugs to market. In particular, they are looking at data science and AI as keys that unlock the value of their data. While you may think of AI as just ChatGPT, which can create cool poems for you, AI is much broader and encompasses capabilities like computer vision, predictive analysis, and machine learning.

AI, when applied appropriately, can provide significant benefits to an organization:
- Increased coverage – you can analyze and leverage much more data than you could have when things were manual. Additionally, you can have more team members use this data because some AI tools replace the need for sophisticated end-user coding and programming.
- Increased efficiency – by using templated algorithms, evolving datasets, and workflows, employees can leverage previous corporate efforts and reduce the amount of work they need to perform to get the answers they want.
- Improved accuracy – by reducing the novel/new work that people perform to arrive at a decision, you are reducing the opportunities for errors to be introduced into the decision.
These benefits are compelling for all industries, but AI has an opportunity to improve the current state of drug development significantly.
AI and Data Science in R&D
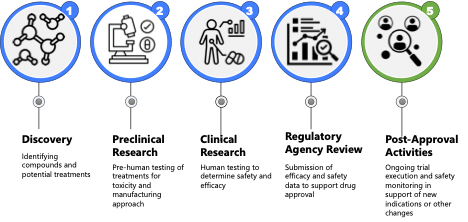
As mentioned earlier, bringing a treatment to market requires lots of teams going through numerous stages and phases of R&D. In the section below, I am going to highlight some areas where Life Sciences companies are using AI and Data Science to improve the journey to approval.
Discovery and Preclinical
All treatments begin with scientists looking for treatment candidates for conditions – it is a two-piece puzzle where they are looking to understand the target and then identify potential candidates. In the very early days of pharma, it was more guesswork, but as science improved, we moved to a place where protein structure and genomic information guided scientists in both target and molecule identification. This often involves looking through a vast library of previously identified molecules and synthesizing new molecules.
- Target identification – Companies are using data science to do wide-ranging omics statistical analysis, using machine learning to predict which genes could be a target, and analyzing the “druggability” of a target by evaluating binding sites on cells.
- Lead molecule identification– Companies are using machine learning algorithms to analyze huge datasets of cell/compound structures to identify potential drug candidates. Companies are also using genomic data to identify potential treatments.
Once candidates are identified, companies look to use AI and Data Science to get to “Is it safe and effective?” faster. Preclinical focuses on understanding how the drug interacts with the body and determining safety. AI allows us to engage in predictive modeling, which uses datasets and algorithms to model pharmacodynamics and pharmacokinetics. We have a large universe of knowledge, and AI allows us to better understand molecular actions (and possibly forecast toxicity) and use genomic data to predict pharmacodynamics.
As you can hopefully see, AI and Data Science have the potential to make us more efficient in identifying targets and candidate molecules. This can be a great way to start our journey to higher rates of success – and make us view the 9.6% success rate as a relic of the past.
Clinical Research
Clinical trials are an area where we can extract immense value from data using data science and AI because we run so many trials (over 85K trials on clinicaltrials.gov are recruiting or actively being conducted) and because trials are so long and expensive (a Phase III trial can range from $25 to $200 million.) When you combine this with the failure rate of trials (approximately 40% of cancer trials are terminated early), the need for change is obvious. We have a great opportunity to drive improvements that will significantly impact the industry and patients.
- Study design – Starting with an appropriate study design can improve success by ensuring we are able to recruit patients and wind up with data that will support our submission for approval. Companies are using AI to glean insights from abstracts and registries to understand the competitive landscape of trials that are being run. Understanding how others are targeting endpoints, using technology (ePRO, sensors, etc), and defining inclusion/exclusion criteria allows us to optimize trial design. Additionally, we can identify opportunities to reduce site burden by reducing non-essential procedures.
- Site identification – Finding the right sites is critical to trial success because they are the conduit to patients and are responsible for the study execution, which generates the data we need to evaluate the treatment candidate. Companies are turning to algorithms and data analysis to look at past study performance and access to patients to identify potential sites and reduce the feasibility/selection effort.
- Subject recruitment – Patient recruitment consumes one-third of the average study duration, so anything we can do here significantly impacts overall trial timelines. In addition to being time-consuming, current patient recruitment approaches result in widespread failure (80% of sites fail to meet their enrollment goals) that has to be overcome by Herculean efforts of other sites and the study team. To address these challenges, some companies are using data science to evaluate eligibility criteria, which can drastically impact recruitment/enrollment efforts; others are using data science to translate inclusion/exclusion criteria into a database query to look into EMR systems to assist sites, and other companies are creating digital twins of subjects to see how they would do in both control and experimental groups. All of these approaches have the potential to reduce the impact of enrollment challenges and shortfalls.
- Subject management– One key use case for technology is searching for a way to retain patients for a study. It may seem like patients would want to continue in a trial, but sadly, 30% of patients drop out of a trial after enrollment. Companies are using AI to look at past dropout indicators (missed appointments, lack of medication adherence, postings on social media, etc) to identify patients who may drop out and then proactively work to retain them.
- Risk and Site Management – Companies have started using access to operational data to support the adoption of risk-based monitoring. In fact, recent analysis shows that 22% of trials use at least one RBM component (KRIs, centralized monitoring, remote monitoring, reduced SDV, and reduced SDR). Improved algorithms and comfort with AI and data science could cause these numbers to jump in the future. Additionally, many companies (and their clinical vendors) are leveraging AI to support improved TMF management by auto-classifying content using algorithms to guide the determination of inspection readiness.
- Study data management – Data management is a very complex arena that requires a lot of thought and a lot of manual work. Some companies are using AI to auto-generate CRFs from the protocol to reduce the time needed for study builds. Companies are also using data science to streamline the query process that Sponsors rely on to question/verify data that was provided by a site.
- Safety monitoring – As we have discussed previously, a key component of clinical research is to confirm the safety of a treatment. Adverse events are collected by sites and going through intake and triage, and companies are using AI and automation to streamline this process. Additionally, some companies pull data from EMR/insurance systems to glean potential adverse events and pull data from social media to understand patient issues/feelings.
FDA Drug Review
As you can imagine, clinical trials generate copious amounts of data, and much of this data is required to support an NDA regulatory submission. However, the data generated via EDC or other sources is not what regulators want to see. They want to see an analysis of the data regarding safety and efficacy. This analysis requires significant effort from the biostats teams and is an area that Life Sciences companies have been looking to streamline. Leveraging a robust SCE that contains reusable templates, workflows, traceability, and other components allows teams to focus on the analysis and less on the mechanics of preparing the data.
Additionally, pharma companies are experimenting with GenAI to draft clinical study reports based on data from the SCE or other data sources. This drafting is possible because of how templated these documents need to be due to regulatory requirements.
Benefits of AI and Data Science in R&D
As you can hopefully see, there are many processes or capabilities that the Life Sciences industry is looking at Data Science and AI to improve – and for good reason. AI has been used in other industries for a long time and has generated improvements in productivity, risk avoidance, and quality. It is time for Life Science to more fully embrace the potential of their data and data science/AI.
I truly believe that if we apply AI across the discovery and development lifecycle, we will see improvements in how we bring treatments to market.
- Discovery will be optimized because AI and our vast collection of data will allow us to identify targets and candidates more efficiently.
- These more carefully selected candidates will proceed through clinical trials faster because we will be addressing key pain points (study design, site identification, patient recruitment, and risk management).

In summary, if we use our data better in the industry, we will be spending money more wisely and bringing treatments to market faster, which has a financial impact on the industry and an outcomes impact on the patients who desperately are looking for a brighter future. In future posts, I will dive deeper into the individual use cases and how AI and data science can help us improve development execution.

